Overall Rating: 2.3/5.0 stars
⚠️ CONSUMER CAUTION ADVISED ⚠️
AZO Yeast Plus presents a complex case study in homeopathic medicine marketing. While positioned as the “#1 Vaginal Health Brand,” this homeopathic remedy faces significant scientific scrutiny and raises important questions about efficacy and safety that American consumers should carefully consider before purchasing.

Packaging of AZO Yeast Plus Dual Relief tablets showing symptom relief benefits and product details azoproducts
Executive Summary
After comprehensive analysis of AZO Yeast Plus, including regulatory status, clinical evidence, consumer feedback, and pricing across US retailers, this review reveals concerning gaps between marketing claims and scientific support. The product’s homeopathic formulation, controversial ingredient selection, and lack of FDA approval for effectiveness create substantial uncertainty about its therapeutic value for American women seeking yeast infection relief.
Product Overview & Key Claims
Manufacturer: i-Health, Inc. (subsidiary of dsm-firmenich)
Product Type: Homeopathic medicine
Primary Claims: Relief from vaginal yeast infection symptoms including itching, burning, odor, and discharge
Package Size: 60 tablets
Dosage: 1 tablet orally, 3 times daily during symptoms
Understanding Yeast Infections
Vaginal yeast infections affect approximately 75% of women at least once in their lifetime, with symptoms that can significantly impact quality of life. Understanding these symptoms is crucial for proper treatment selection.

Common symptoms of vaginal yeast infection include itching, burning, redness, pain, rash, and abnormal vaginal discharge redboxrx
Ingredient Analysis & Formulation Concerns
Active Ingredients (Per Tablet)
- Candida albicans 30X – The actual yeast that causes infections
- Kreosotum 30X – Wood creosote derivative
- Natrium muriaticum 12X – Homeopathic salt (sodium chloride)
- Sulphur 12X – Chemical element sulfur
Critical Ingredient Concern: Candida albicans Inclusion
The most troubling aspect of AZO Yeast Plus is its inclusion of Candida albicans 30X – literally the same organism that causes yeast infections. This counterintuitive formulation follows homeopathic “like cures like” principles, but medical experts question the logic of introducing the pathogen responsible for the infection, even in diluted form12.
Homeopathic Dilution Process
The “30X” notation indicates the ingredients have been diluted 30 times in a 1:10 ratio, resulting in concentrations so minimal that virtually no molecules of the original substance remain in the final product.

Step-by-step process of homeopathic nosode preparation illustrating dilution, trituration, and succussion stages labeled by centesimal potencies (1CH to 200CH) mdpi
Scientific Evidence & Effectiveness Assessment
Lack of Clinical Support
No Specific Studies: AZO’s own FAQ acknowledges that “no studies have been done to determine if it will cure an active vaginal or yeast infection”34. This admission significantly undermines confidence in the product’s therapeutic claims.
Homeopathic Medicine Controversy
Multiple systematic reviews and meta-analyses have found homeopathic treatments to be no more effective than placebo:
- A 2000 European study concluded homeopathy shows “low strength of evidence” due to poor methodological quality of trials5
- The FDA has expressed concerns that it “cannot ensure these drugs meet standards for safety, effectiveness, and quality”6
- Medical consensus considers homeopathic dilutions to be scientifically implausible7
Comparison with Proven Treatments
Evidence-based yeast infection treatments show significantly higher success rates:
- Fluconazole (Diflucan): 70-88% clinical cure rates in controlled trials8
- Miconazole (Monistat): >90% effectiveness for clinical and mycological cure9
- Topical antifungals: Consistently demonstrate superior efficacy compared to homeopathic alternatives10
Safety Profile & Side Effects
Positive Safety Aspects
- Generally well-tolerated due to minimal active ingredient concentrations
- No significant drug interactions reported
- Safe for most adults when used as directed
Potential Concerns
- Delayed Treatment: Relying on ineffective remedies may postpone proper medical care
- Misdiagnosis Risk: Self-treating without professional diagnosis can mask other conditions
- Oral Thrush Reports: Some users report developing yeast infections in the mouth after use1
FDA Safety Warnings
The FDA requires all homeopathic products to carry disclaimers acknowledging lack of evaluation for safety or effectiveness.

Drug facts label of a homeopathic product detailing active ingredient, uses, warnings, and FDA disclaimer npr
Consumer Experience & Reviews
Mixed Customer Feedback
Amazon Reviews: 4.5/5 stars from over 17,000 reviews, indicating general customer satisfaction despite scientific concerns1
Positive Reports:
- Some users report symptom relief within days
- Convenient oral tablet form
- Affordable compared to prescription alternatives
Negative Experiences:
- No symptom improvement in some cases
- Development of oral yeast infections
- Complaints about product ineffectiveness1112
Healthcare Provider Perspectives
Medical professionals generally recommend evidence-based treatments over homeopathic alternatives, citing lack of proven efficacy and potential for delayed appropriate care13.
Pricing Analysis Across US Retailers
Pricing for AZO Yeast Plus varies significantly across major US retailers, with differences of up to 83% between the highest and lowest prices.
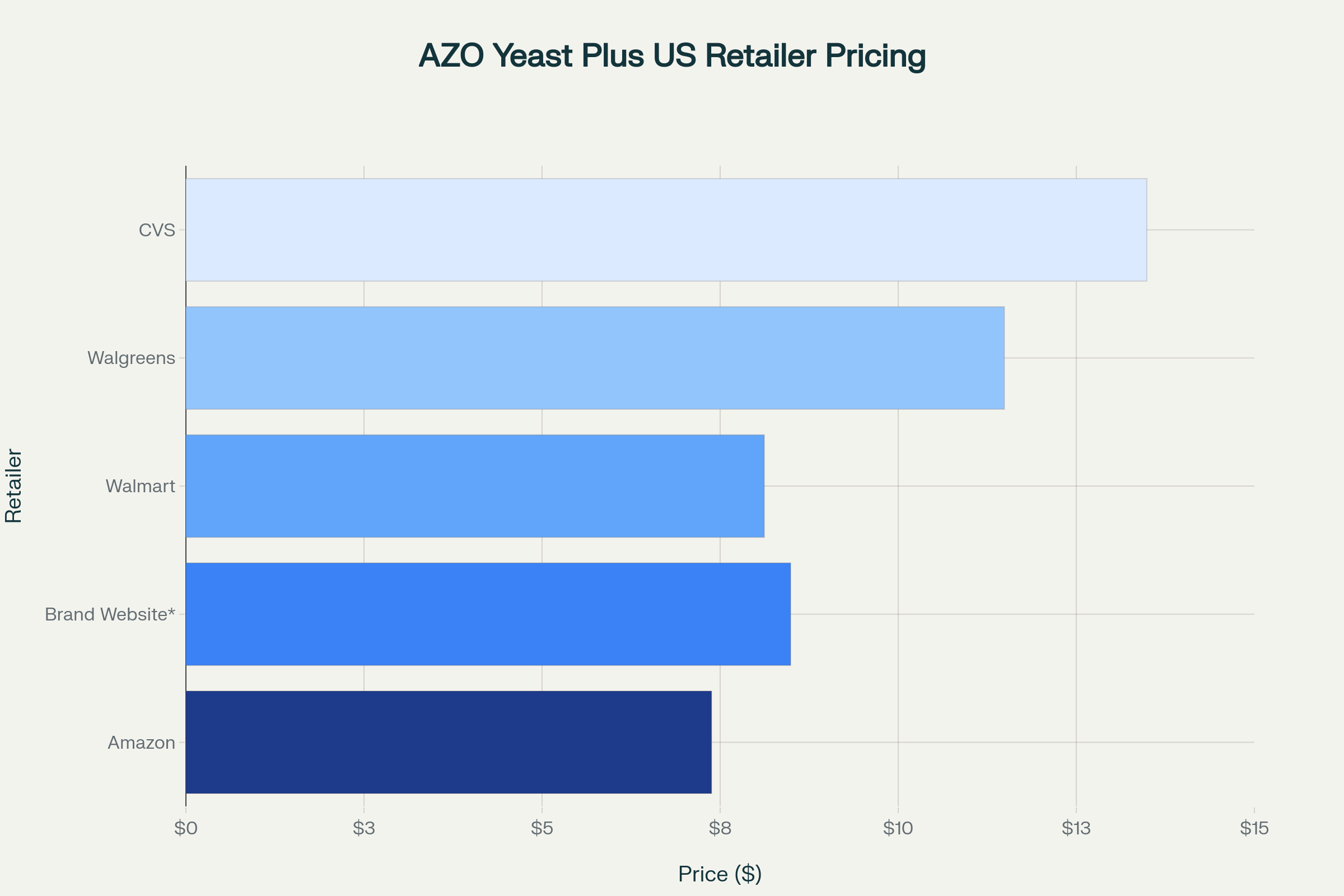
Price comparison of AZO Yeast Plus across major US retailers, showing significant variation in cost
Best Value: Amazon at $7.38 (includes free shipping)
Highest Price: CVS at $13.49
Average Price: $9.79 across surveyed retailers
Regulatory Status & FDA Position
Current Regulatory Framework
AZO Yeast Plus operates under FDA’s homeopathic drug guidance, which allows marketing without pre-approval but requires specific labeling disclaimers614:
- Must state the product has not been evaluated by FDA
- Cannot claim to cure, treat, or prevent specific diseases
- Must include appropriate warnings and dosage instructions
FDA Risk-Based Enforcement
The FDA has indicated it will prioritize enforcement actions against homeopathic products that:
- Pose significant safety concerns
- Target vulnerable populations
- Make unsupported disease treatment claims
Alternative Treatment Options
Evidence-Based Alternatives
Over-the-Counter Options:
- Monistat (miconazole): 1, 3, or 7-day treatments with proven efficacy
- Clotrimazole creams: Available at lower cost with clinical support
- Boric acid suppositories: Effective for resistant infections
Prescription Options:
- Fluconazole (Diflucan): Single-dose oral treatment with high success rates
- Ibrexafungerp (Brexafemme): Newer oral antifungal for resistant cases
Natural Approaches with Some Evidence
- Probiotics: Clinical studies show benefit as adjunctive therapy15
- Boric acid: Established antifungal properties with medical support16
Market Context & Industry Analysis
The US women’s health supplement market is projected to reach $77.46 billion by 2030, with increasing demand for “natural” alternatives driving growth despite limited scientific validation17. This trend creates opportunities for products like AZO Yeast Plus to capture market share through consumer preference for perceived natural solutions.
Recommendations for US Consumers
❌ Not Recommended As Primary Treatment
- Seek Professional Diagnosis: Consult healthcare providers for accurate diagnosis before self-treating
- Choose Evidence-Based Options: Select treatments with proven clinical efficacy
- Consider Homeopathic Limitations: Understand the scientific consensus on homeopathic medicine
- Monitor for Improvement: If symptoms persist beyond 3 days, seek medical attention
⚠️ If You Choose to Try AZO Yeast Plus
- Use as complementary, not primary treatment
- Maintain realistic expectations about effectiveness
- Monitor symptoms closely and seek medical care if no improvement
- Purchase from reputable retailers to ensure product authenticity
- Be aware of the 60-day return policies at most major retailers
Final Verdict
Rating: 2.3/5.0 stars
AZO Yeast Plus represents a concerning example of marketing success over scientific validation. While generally safe due to its homeopathic dilutions, the product’s effectiveness claims lack credible scientific support, and its inclusion of the actual yeast pathogen raises logical questions about its formulation rationale.
For American consumers seeking yeast infection relief, established antifungal treatments offer superior efficacy, faster symptom resolution, and solid scientific backing. The significant price variations across retailers (ranging from $7.38 to $13.49) also suggest market inefficiencies that savvy consumers should navigate carefully.
Bottom Line: While AZO Yeast Plus may provide placebo benefits for some users, women experiencing yeast infection symptoms deserve treatments backed by robust clinical evidence. The medical consensus strongly favors proven antifungal medications over homeopathic alternatives for this common but treatable condition.
This review is based on publicly available information, regulatory filings, clinical research, and consumer feedback as of July 2024. Always consult healthcare professionals before starting any new treatment regimen.
- https://trends.google.com/trends/explore?q=azo+yeast+plus+review&date=today+1-m&geo=US
- https://amzn.to/3UwhcyF
- https://illuminatelabs.org/blogs/health/azo-yeast-plus-review
- https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=22845cac-8011-4ea8-b35e-b72973eddb44
- https://www.hdis.com/azo-yeast-infection-relief
- https://www.woot.com/review/B0D5FZGY8W?itemid=WT491612A&offerid=56b93999-8546-4dee-94a0-c6de3669ade5&filter=5
- https://www.walmart.com/reviews/product/167682177
- https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?audience=consumer&setid=22845cac-8011-4ea8-b35e-b72973eddb44
- https://dailymed.nlm.nih.gov/dailymed/getFile.cfm?setid=22845cac-8011-4ea8-b35e-b72973eddb44&type=pdf
- https://casadesante.com/blogs/wellness/azo-yeast-plus-review
- https://azoproducts.com/products/azo-yeast-plus?bvstate=pg%3A2%2Fct%3Ar
- https://azoproducts.com/products/azo-yeast-plus
- https://www.webmd.com/drugs/2/drug-181172/azo-dual-protection-oral/details
- https://fsastore.com/azo-yeast-natural-symptom-prevention-and-relief-400-mg-tablets-60-ct./6680.html
- https://www.empr.com/drug/azo-yeast/
- https://www.target.com/p/azo-yeast-plus-dual-relief-yeast-infection-vaginal-symptom-relief-60ct/-/A-11063211
- https://mms.mckesson.com/product/1065928/I-Health-Inc-87651060667
- https://in.iherb.com/r/azo-yeast-plus-60-tablets/12980
- https://www.hy-vee.com/aisles-online/p/34522/AZO-Yeast-Plus-MultiBenefit-Homeopathic-Medicine-Tablets
- https://www.walmart.com/reviews/product/10317752
- https://in.iherb.com/pr/azo-yeast-plus-60-tablets/12980
- https://www.heb.com/product-detail/azo-yeast-plus-yeast-infection-relief-tablets/466925
- https://www.linkedin.com/company/i-health-inc.
- https://www.drugs.com/azo.html
- https://pubmed.ncbi.nlm.nih.gov/10853874/
- https://rocketreach.co/i-health-inc-a-division-of-dsm-firmenich-profile_b4440961fab4c472
- https://www.drugs.com/sfx/azo-hormonal-health-side-effects.html
- https://www.goodrx.com/conditions/yeast-infection/monistat-vs-diflucan-fluconazole-which-is-better-for-a-yeast-infection
- https://pmc.ncbi.nlm.nih.gov/articles/PMC1668980/
- https://www.prnewswire.com/news-releases/i-health-inc-announces-agreement-to-purchase-up4-probiotics-300399766.html
- https://www.goodrx.com/phenazopyridine/common-side-effects
- https://www.drugs.com/drug-class/topical-antifungals.html
- https://www.ccrhindia.nic.in/index1.aspx?lsid=4679&lev=2&lid=2619&Regid=0&langid=1
- https://www.azo.com/en-de/company/history
- https://www.1mg.com/medicines/azo-46657
- https://www.mayoclinic.org/diseases-conditions/vaginitis/expert-answers/yeast-infection-during-pregnancy/faq-20058355
- https://jamanetwork.com/journals/jamadermatology/fullarticle/189547
- https://www.biospace.com/b-i-health-inc-b-release-new-azo-product-addresses-bladder-control-in-two-ways
- https://www.webmd.com/drugs/2/drug-168446-86/azo-urinary-pain-relief/details
- https://sesamecare.com/blog/yeast-infection-treatments
- https://www.sciencedirect.com/science/article/pii/S0965229924000967
- https://www.walmart.com/ip/AZO-Yeast-Plus-Dual-Relief-Tablets-FSA-HSA-Eligible-Relieves-Itching-Burning-60-Count/10317752
- https://www.singlecare.com/blog/vagisil-vs-monistat/
- https://www.verywellhealth.com/home-remedies-for-yeast-infections-5176162
- https://www.grandviewresearch.com/industry-analysis/women-health-beauty-supplements-market
- https://www.walgreens.com/store/c/productlist/azo-feminine-treatments/N=361315-2218
- https://hsastore.com/monistat-1-day-yeast-infection-treatment/41400.html
- https://www.medicalnewstoday.com/articles/317935
- https://media.market.us/womens-health-and-beauty-supplements-market-news-2025/
- https://www.walgreens.com/store/c/productlist/N=2218/1/Brands=yes
- https://azoproducts.com/products/azo-yeast-plus?bvstate=pg%3A4%2Fct%3Ar
- https://khealth.com/learn/yeast-infection/home-remedies/
- https://finance.yahoo.com/news/women-health-beauty-supplements-market-153300529.html
- https://www.walgreens.com/store/c/productlist/yeast-infection-treatment-medications-and-treatments/N=361315-2000011396
- https://www.redboxrx.com/blog/fluconazole-diflucan-vs-miconazole-monistat-which-is-best-for-yeast-infections
- https://www.webmd.com/women/remedies-yeast-infections
- https://www.futuremarketinsights.com/reports/womens-health-supplement-market
- https://www.walgreens.com/store/c/productlist/N=2218-9000157753-4294896499
- https://health.usnews.com/otc/rankings/yeast-infection-prevention-and-relief
- https://www.goodrx.com/conditions/yeast-infection/whats-the-best-way-to-treat-a-yeast-infection-fast
- https://www.researchandmarkets.com/report/womens-health-supplement
- https://www.hoganlovells.com/en/publications/homeopathic-drug-product-makers-warned-to-follow-fda-premarket-and-gmp-rules
- https://www.lemon8-app.com/@_litxeyes/7377183333136089616?region=us
- https://pmc.ncbi.nlm.nih.gov/articles/PMC9921437/
- https://www.accc.gov.au/about-us/news/media-updates/textiles-recalled-after-tests-for-azo-dyes
- https://www.science.org/content/article/fda-takes-new-look-homeopathy
- https://www.backyardchickens.com/threads/azo-pills.1536495/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC2364408/
- https://www.accc.gov.au/media-release/accc-testing-prompts-clothing-recalls
- https://www.thefdalawblog.com/2024/10/homeopathic-industry-group-wants-court-to-exclude-it-from-fdas-enforcement-plans/
- https://liveutifree.com/recurrent-urinary-tract-infections/
- https://www.scirp.org/journal/paperinformation?paperid=87803
- https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan
- https://lexcomply.com/blog/drugs-fifth-amendment-rules-2024-licenses-to-manufacture-or-sale-of-homeopathic-medicines/
- https://www.reddit.com/r/Interstitialcystitis/comments/1anzdqa/azo_yeast_and_azo_urinary/
- https://academic.oup.com/cid/article/38/2/161/286280
- https://www.azo-inc.com/blog/9-material-handling-stories-you-almost-missed-from-august
- https://www.fda.gov/drugs/information-drug-class/homeopathic-products
- https://www.goodrx.com/drugs/side-effects/is-your-medication-causing-hair-loss-these-drugs-are-common-culprits
- https://www.sciencedirect.com/science/article/abs/pii/S0002937825001772
- https://www.satra.com/bulletin/article.php?id=959
- https://www.fda.gov/news-events/press-announcements/fda-issues-warning-letters-firms-marketing-unapproved-eye-products
- https://health.clevelandclinic.org/do-home-remedies-actually-work-for-yeast-infections
- https://www.healthline.com/health/allergies/yeast
- https://azoproducts.com/products/azo-yeast-plus?bvstate=pg%3A3%2Fct%3Ar
- https://www.ftc.gov/news-events/news/press-releases/2020/03/ftc-fda-send-warning-letters-seven-companies-about-unsupported-claims-products-can-treat-or-prevent
- https://pmc.ncbi.nlm.nih.gov/articles/PMC11012191/
- https://balanceone.com/blogs/news/top-7-antifungal-supplements-to-fight-candida
- https://pmc.ncbi.nlm.nih.gov/articles/PMC3769763/
- https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/az-pharmaceutical-inc-645101-06012023
- https://www.mayoclinic.org/diseases-conditions/yeast-infection/diagnosis-treatment/drc-20379004
- https://pmc.ncbi.nlm.nih.gov/articles/PMC9006731/
- https://www.healthline.com/health/womens-health/boric-acid-for-yeast-infection
- https://www.nasc.cc/members-health/fda-warning-letters-sent-to-pet-product-companies/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC7711718/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC7151124/
- https://www.fda.gov/drugs/drug-supply-chain-integrity/internet-pharmacy-warning-letters
- https://www.ncbi.nlm.nih.gov/books/NBK459317/
- https://www.healthline.com/nutrition/candida-diet
- https://www.medicalnewstoday.com/articles/325518
